An electron highway headed for methanol
Making methanol just got a lot easier, now that chemists at Yale have opened up a new electron highway.
The discovery, published online Nov. 27 in the journal Nature, finds a novel solution for two chemical tasks: producing methanol—a volatile, liquid fuel that is prized by industry—and removing carbon dioxide from the atmosphere. Hailiang Wang, an assistant professor of chemistry at Yale and member of the Energy Sciences Institute at Yale's West Campus, led the research.
Methanol is used in a variety of products, including antifreeze, paint thinners, and glass cleaners. It is also used to produce biodiesel fuel, plastics, plywood, and permanent-press clothing.
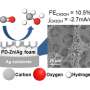
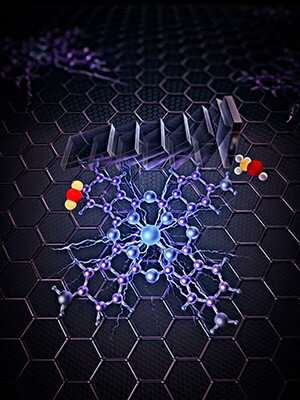
Yale researchers developed a catalyst that converts carbon dioxide and water into methanol using electricity. It's a type of catalyst called a heterogeneous molecular electrocatalyst—"heterogeneous" because it's a solid catalyst material operating in a liquid electrolyte, and "molecular" because the active site of the catalyst is a molecular structure.
The distinct structure of the new catalyst is the key, Wang said.
He and his team anchored individual molecules of cobalt phthalocyanine (or its derivative) onto the surface of carbon nanotubes, nanometer-sized tubes of rolled up graphene layers. The nanotubes act like a highway for electrons, creating a rapid and continuous delivery of electrons to the catalytic sites for converting carbon dioxide to methanol. It is a six-electron reduction process, the researchers said, meaning that six electrons are injected into one carbon dioxide molecule.

Prior to this discovery, a more limited delivery of electrons—a two-electron reduction process—meant molecular catalysts were only able to convert carbon dioxide into products such as carbon monoxide.
"Heterogenized molecular catalysts allow our group to do new chemistry and known chemistry in better ways, and this is one example," Wang said.
Yueshen Wu, a graduate student at Yale, is first author of the study. Co-authors are postdoctoral fellow Xu Lu of Yale and associate professor Yongye Liang and graduate student Zhan Jiang of the Southern University of Science and Technology in China.
More information: Yueshen Wu et al. Domino electroreduction of CO2 to methanol on a molecular catalyst, Nature (2019). DOI: 10.1038/s41586-019-1760-8
Journal information: Nature
Provided by Yale University