Experiment shows non-classical growth of crystals
This might considerably speed up crystal growth that is of major importance in a number of materials and applications. The liquid state of the building blocks in the preliminary stage might also accelerate the effectiveness of medicines. The results have been published in the current issue of the scientific journal Nature Communications on 21 June 2017.
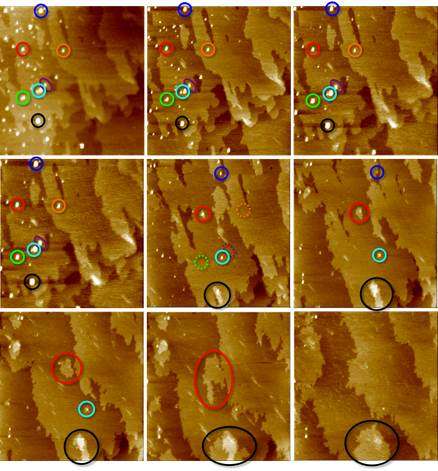
Professor Coelfen's research team used an atomic force microscope for the measurements in this preliminary stage. The images thus obtained show bright spots that become darker as time progresses, and finally amalgamate entirely with the crystal surface. The atomic force microscope translates brightness into height. The brighter the spot, the higher the component which then spreads until it has reached the height of the crystal surface. It now forms a new crystal layer. Helmut Coelfen explains the principle: "If I create a new layer with atoms or molecules, I need a lot of those. If, however, my solution already contains building blocks, I can add many building blocks to the intended building site in one go."
The existence of these nano-drips was known already before the Konstanz experiment. They were found for proteins, which are very large macromolecules. Glutamic acid, in contrast, is a single amino acid, a very small molecule. This non-classical growth in such small molecules was observed for the first time. as was the successful measurement. Strictly speaking, the liquid state has not yet been proven, but is concluded from the building blocks' property on the crystal surface. "We think it must be liquids, otherwise the nano-drips would not spread in that way", says Helmut Coelfen.
If glutamic acid uses this mechanism of liquid preliminary stages to grow, this might also apply to other molecules. Helmut Coelfen has particularly new formulations for active substances in medicines in mind. As liquids dissolve faster than solid matters, such medicines would become effective much faster. The experiment of the Coelfen research team can also measure the rate at which the layers grow and thus calculate how many building blocks the liquid contains. "This contributes to a fundamental understanding of crystal growth", says Coelfen. Deviations of expected crystal growth can also be explained through this observation.
New physical-chemical theories of crystal growth will now have to be developed to theoretically describe the empirical observation of the liquid preliminary stage. The crucial questions are: Where do these small building blocks come from? Why do they turn liquid? And why can they create a crystal layer? Helmut Coelfen's research team has provided the experimental material for the theory to come.
More information: Yuan Jiang et al, Growth of organic crystals via attachment and transformation of nanoscopic precursors, Nature Communications (2017). DOI: 10.1038/ncomms15933
Journal information: Nature Communications
Provided by University of Konstanz





















